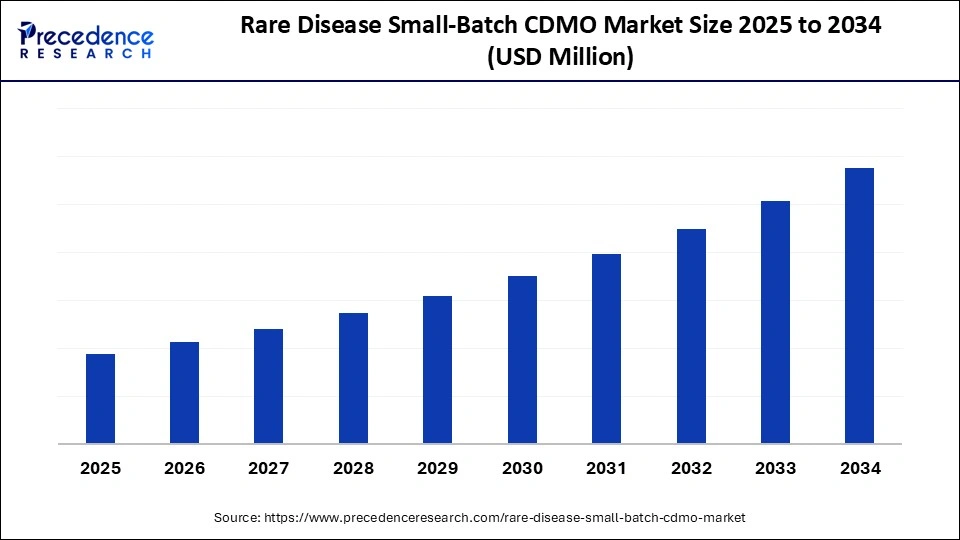
The rare disease small-batch CDMO market is projected to experience significant growth through 2034, driven by the increasing prevalence of rare diseases and the rising approvals of orphan drugs. With an expected compound annual growth rate (CAGR) propelled by advancements in precision medicine and specialized manufacturing, this market is becoming a critical partner for biopharma innovators focused on delivering niche and personalized therapies.
Rare Disease Small-Batch CDMO Market Key Insights
-
The market is currently led by North America, the dominant region in rare disease small-batch CDMO services.
-
Asia Pacific is the fastest-growing region, fueled by expanding healthcare investments and biotechnology capabilities.
-
Clinical scale API and drug product manufacturing are the backbone service types in this sector.
-
Small molecules remain the dominant modality, while viral vectors and gene therapies represent the fastest-growing technologies.
-
Genetic and neurological rare disease programs are the main therapeutic focus.
-
Small biotech and startup companies form the largest customer segment.
-
Fee-for-service and fixed-price contract models maintain market dominance, with risk-sharing agreements emerging.
-
Leading companies include CDMOs specializing in gene therapy, RNA therapeutics, and analytical fill-finish services.
Market Growth and Key Drivers
The rare disease small-batch contract development and manufacturing organization (CDMO) market has gained remarkable traction due to the surge in orphan drug approvals and a growing pipeline of precision therapies targeting rare conditions. Small-batch CDMOs offer the flexibility, specialized technologies, and regulatory expertise critical for the manufacture of low-volume, high-value products including small molecules, biologics, cell and gene therapies, and advanced therapies medicinal products (ATMPs). These factors underpin a compelling CAGR forecast for the market as innovation accelerates and collaborations between biotech firms and CDMOs deepen.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6767
How is Artificial Intelligence Transforming the Market?
Artificial intelligence (AI) plays a transformative role by optimizing small-batch design, testing, and manufacturing operations. Predictive analytics help anticipate production bottlenecks and optimize resource allocation, leading to quicker turnaround times and reliability improvements. AI-driven quality assurance detects anomalies, reducing batch failure risks that are especially costly in rare disease batches.
Moreover, AI-powered digital twin simulations enable virtual replication of small-scale manufacturing runs, optimizing process parameters before physical execution. AI also streamlines supply chain management by coordinating raw material procurement efficiently, thereby lowering costs and shortening time-to-market for rare disease therapies. These advanced technologies elevate confidence across the development and manufacturing value chain.
What Are the Core Growth Factors?
The growth of the rare disease small-batch CDMO market is fueled by:
-
Increasing prevalence and diagnosis of rare diseases worldwide.
-
Government incentives such as tax credits and market exclusivity for orphan drugs.
-
Rising investments in RNA-based therapies, gene editing, and precision medicine.
-
The necessity for agile, customized manufacturing platforms.
-
Expansion of clinical and commercial small-batch manufacturing to meet pipeline demands.
-
Shifts towards virtual biotech models outsourcing nearly all development functions.
Which Emerging Trends and Opportunities Define the Market?
What is driving the rise of rare oncology projects in the CDMO niche?
Rare oncology therapies, including precision medicine and immuno-oncology, are leading the fastest-growing therapeutic segment. This trend reflects unmet medical needs in rare cancers and advances in targeted therapies supported by CDMOs specializing in complex manufacturing.
How are Asia Pacific markets influencing global CDMO dynamics?
Asia Pacific is rapidly ascending as a growth hub due to increased healthcare spending, government policy support, clinical trial expansion, and strategic foreign investments in biotechnology. Adoption of AI and automation further accelerates market momentum in this region.
What are the implications of virtual biotech companies for CDMO services?
Virtual biotechs lean companies outsourcing nearly all activities—drive demand for integrated CDMO service portfolios. This model enhances speed and cost efficiency, a crucial advantage in rare disease drug development.
Regional and Segment Analysis: What Are the Market Nuances?
North America remains the market leader, supported by its established biotech ecosystem, funding, and favorable orphan drug regulations. The Asia Pacific is the fastest-growing region with emerging capabilities and cost advantages fostering new CDMO investments. Europe follows with steady growth driven by regulatory harmonization and innovation clusters.
Service types are dominated by clinical-scale API and drug product manufacturing, followed by rapid expansion in analytical development and specialized aseptic fill-finish services, critical for injectable biologics and gene therapies.
Small molecules lead the modality segment due to legacy infrastructure and cost-effectiveness, while viral vector gene therapies present the highest growth rates, aligned with curative genetic approaches.
Therapeutic areas focus on genetic and neurological disorders given the high genetic basis of many rare diseases. Rare oncology is emerging rapidly due to precision immunotherapies addressing unmet needs.
Customer types are primarily small biotechs and startups relying on CDMOs for flexible access to manufacturing and regulatory compliance, with virtual biotechs expanding swiftly.
Contract models are predominantly fee-for-service and fixed-price, with risk-sharing agreements gaining traction as development complexity grows.
Market Challenges and Cost Pressures
The small-batch CDMO market faces scaling challenges due to limited rare disease expertise, high operational costs of GMP-compliant small-scale facilities, and tight regulatory scrutiny. Supply chain fragility for rare raw materials and lower commercial returns relative to large-scale manufacturing add pressure. Profitability remains a hurdle as CDMOs balance quality, speed, and cost containment.
Rare Disease Small-Batch CDMO Market Companies

- Lonza.
- Catalent.
- Thermo Fisher Scientific / Patheon.
- WuXi Biologics / WuXi AppTec.
- Samsung Biologics.
- FUJIFILM Diosynth Biotechnologies.
- Ajinomoto Bio-Pharma Services (ABP)
- BioVectra
- Alcami.
- CordenPharma
- Charles River Laboratories
- Novasep / Combi-blocks
- Aenova / Vetter
- PCI Pharma Services
- Jubilant Biosys / Jubilant HollisterStier
Case Study: Accelerating Gene Therapy Supply
A leading CDMO recently partnered with a biotech startup to ramp up a viral vector gene therapy program for a rare genetic disorder. By leveraging AI-powered process optimization and flexible small-batch manufacturing, the project shortened development timelines by 30%, ensuring timely clinical trial material delivery under stringent quality standards.
Read Also: Metabolomics Market
You can place an order or ask any questions. Please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344
- Multi-Tenant Data Centers Market Size to Surge USD 189.59 Billion by 2034 - September 15, 2025
- Data Center Containment Market Size to Attain USD 8.10 Billion by 2034 - September 15, 2025
- Rare Disease Small-Batch CDMO Market Accelerate orphan drug production with flexible manufacturing and tailored CDMO partnerships - September 15, 2025
